|
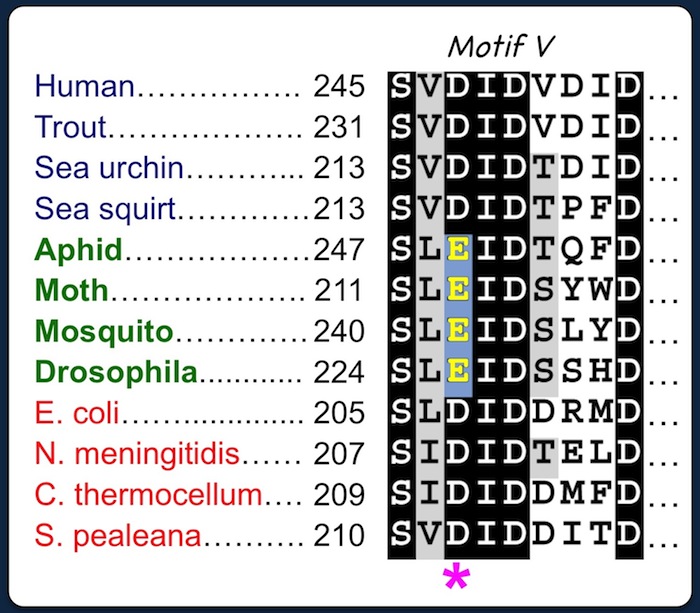
Deference in Motif V suggests that insect CSASs coordinate metal cofactors differently from counterparts in vertebrates and bacteria. |
|
The first aspartic acid of
the DID triad (*) is required for coordinating a catalytic metal cofactor in the active site of bacterial and vertebrate CSAS enzymes. Site-directed mutagenesis suggests that the third D of the EID triad is essential for metal coordination in insect CSAS (Mertsalov et al. 2016). |